Dive into the captivating world of Fiveable AP Chem Unit 3, where intermolecular forces dance and chemical kinetics unravels the secrets of reaction rates. This unit is a cornerstone of AP Chemistry, laying the foundation for understanding the behavior of matter and the dynamics of chemical reactions.
From the interplay of intermolecular forces that shape the properties of substances to the intricate dance of chemical equilibrium, this unit offers a comprehensive exploration of fundamental chemical principles. Prepare to be enthralled as we delve into the depths of Unit 3, unlocking the mysteries of chemistry.
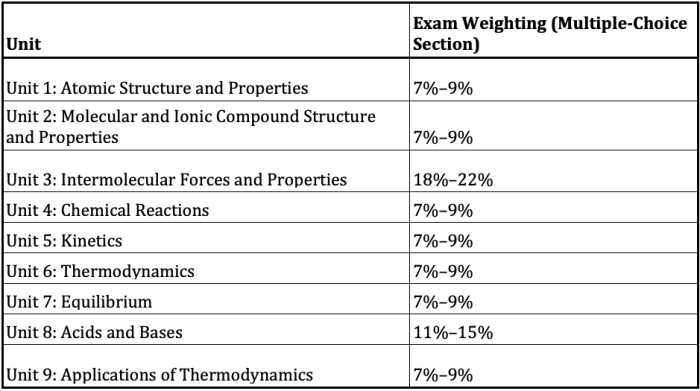
Fiveable AP Chem Unit 3
Unit 3: Intermolecular Forces and Properties is a crucial component of the AP Chemistry curriculum. It delves into the fundamental interactions between molecules, which govern the physical properties and behavior of matter.
This unit explores a range of intermolecular forces, including dipole-dipole interactions, hydrogen bonding, and London dispersion forces. By understanding these forces, students gain insights into why substances exhibit specific properties such as boiling point, melting point, and solubility.
Polarity and Intermolecular Forces
Polarity refers to the uneven distribution of electrons within a molecule. Polar molecules have a positive end and a negative end, which can interact with each other through dipole-dipole forces. The strength of these forces depends on the magnitude of the molecular polarity.
Intermolecular Forces and Properties

Intermolecular forces, the forces between molecules, play a crucial role in determining the physical properties of substances. These forces range from weak to strong and can significantly impact properties such as melting point, boiling point, and solubility.
Types of Intermolecular Forces
There are three main types of intermolecular forces:
- Dipole-dipole forces: These forces occur between polar molecules, where one end of the molecule has a partial positive charge and the other end has a partial negative charge. The oppositely charged ends of different molecules attract each other, forming dipole-dipole interactions.
- Hydrogen bonding: This is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The electronegative atom pulls electron density away from the hydrogen, creating a partial positive charge on the hydrogen and a partial negative charge on the electronegative atom.
These partial charges can then interact with other molecules, forming hydrogen bonds.
- London dispersion forces: These forces are present in all substances, regardless of their polarity. They arise from the temporary fluctuations in electron distribution within molecules. These fluctuations create instantaneous dipoles, which can induce opposite dipoles in neighboring molecules, leading to weak attractive forces.
Impact on Physical Properties
The strength of intermolecular forces directly influences the physical properties of substances:
- Melting point: Substances with strong intermolecular forces, such as hydrogen bonding, have higher melting points. This is because more energy is required to overcome the intermolecular forces and separate the molecules.
- Boiling point: Similar to melting point, substances with strong intermolecular forces have higher boiling points. This is because more energy is required to overcome the intermolecular forces and vaporize the liquid.
- Solubility: Polar substances tend to be more soluble in polar solvents, while nonpolar substances are more soluble in nonpolar solvents. This is because the intermolecular forces between the solute and solvent molecules must be similar for dissolution to occur.
Examples
- Water: Water molecules exhibit strong hydrogen bonding, which results in its high melting point (0 °C) and boiling point (100 °C).
- Methane: Methane molecules have weak London dispersion forces, resulting in its low melting point (-182 °C) and boiling point (-162 °C).
- Sodium chloride: Sodium chloride is an ionic compound that forms strong electrostatic interactions between its ions. These strong intermolecular forces result in its high melting point (801 °C) and boiling point (1465 °C).
Solutions and Colligative Properties
Solutions are homogeneous mixtures composed of two or more components. The component present in the largest amount is called the solvent, while the other components are called solutes.
Solutions can be classified based on their concentration. Dilute solutions have a low concentration of solute, while concentrated solutions have a high concentration of solute. Saturated solutions contain the maximum amount of solute that can be dissolved at a given temperature and pressure.
Colligative Properties, Fiveable ap chem unit 3
Colligative properties are properties of solutions that depend on the concentration of solute particles, not on the identity of the solute. The four main colligative properties are vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
- Vapor pressure lowering: The vapor pressure of a solution is lower than the vapor pressure of the pure solvent. This is because solute particles compete with solvent particles for space at the surface of the solution.
- Boiling point elevation: The boiling point of a solution is higher than the boiling point of the pure solvent. This is because solute particles interfere with the formation of solvent vapor bubbles.
- Freezing point depression: The freezing point of a solution is lower than the freezing point of the pure solvent. This is because solute particles interfere with the formation of solvent crystals.
- Osmotic pressure: Osmotic pressure is the pressure that must be applied to a solution to prevent the flow of solvent molecules from a region of low solute concentration to a region of high solute concentration.
Colligative properties are important in many applications, such as determining the molecular weight of a solute, predicting the behavior of solutions in biological systems, and designing separation techniques.
Chemical Kinetics
Chemical kinetics is the study of reaction rates, the changes in the concentrations of reactants and products with time. Reaction rates are affected by several factors, including the nature of the reactants, their concentrations, the temperature, and the presence of a catalyst.Chemical
reactions occur through different mechanisms, which can impact their rate laws. A rate law expresses the relationship between the reaction rate and the concentrations of the reactants. Understanding reaction mechanisms allows chemists to predict reaction rates and design experiments to control them.
Types of Reaction Mechanisms
There are two main types of reaction mechanisms: elementary reactions and complex reactions. Elementary reactions are one-step processes that occur in a single collision between reactants. Complex reactions involve multiple steps and may include intermediates, which are species that are formed and consumed during the reaction.
Impact of Reaction Mechanisms on Rate Laws
The type of reaction mechanism affects the rate law. For elementary reactions, the rate law is typically first-order or second-order, depending on the number of reactants involved. For complex reactions, the rate law can be more complex and may involve multiple terms.
Applications of Chemical Kinetics
Chemical kinetics is used in various fields, including chemistry, engineering, and medicine. It helps in understanding and predicting the behavior of chemical reactions, designing efficient chemical processes, and developing new drugs and therapies.
Chemical Equilibrium: Fiveable Ap Chem Unit 3
Chemical equilibrium is a state in which the concentrations of reactants and products in a chemical reaction remain constant over time. This means that the forward and reverse reactions are occurring at the same rate, and there is no net change in the concentrations of the reactants and products.The
concept of dynamic equilibrium is central to understanding chemical equilibrium. In a dynamic equilibrium, the forward and reverse reactions are constantly occurring, but the net change in concentrations is zero. This is because the rate of the forward reaction is equal to the rate of the reverse reaction.
The equilibrium constant is a crucial concept in fiveable ap chem unit 3. It helps us understand the extent to which a reaction proceeds and the position of equilibrium. In the same vein, the queen of the south oes plays a pivotal role in her kingdom, maintaining balance and stability.
Just as the equilibrium constant provides insights into chemical reactions, understanding the queen’s influence deepens our comprehension of the intricate dynamics of fiveable ap chem unit 3.
Acids and Bases

Acids and bases are substances that exhibit characteristic properties and undergo specific reactions. They play crucial roles in various chemical and biological processes.
Arrhenius Theory
The Arrhenius theory, proposed by Svante Arrhenius, defines acids as substances that dissociate in water to produce hydrogen ions (H+), while bases dissociate to produce hydroxide ions (OH-).
Brønsted-Lowry Theory
The Brønsted-Lowry theory extends the Arrhenius theory by defining acids as proton donors and bases as proton acceptors. According to this theory, an acid-base reaction involves the transfer of a proton from the acid to the base.
Lewis Theory
The Lewis theory defines acids as electron-pair acceptors and bases as electron-pair donors. This theory provides a more general framework that encompasses both proton transfer and other types of acid-base reactions.
Properties of Acids and Bases
- Acids are typically sour, corrosive, and react with metals to produce hydrogen gas.
- Bases are typically bitter, slippery, and react with acids to produce water and a salt.
Acid-Base Reactions
Acid-base reactions involve the neutralization of an acid by a base. These reactions typically result in the formation of a salt and water.
Applications of Acids and Bases
- Acids are used in batteries, fertilizers, and metalworking.
- Bases are used in cleaning products, soaps, and paper manufacturing.
- Acid-base reactions are essential in many biological processes, such as digestion and respiration.
Applications of Unit 3 Concepts
The concepts covered in Unit 3 have wide-ranging applications in various fields. Intermolecular forces play a crucial role in materials science and drug design, while chemical kinetics finds use in industrial processes and environmental monitoring. Understanding acids and bases is essential in fields like medicine and agriculture.
Intermolecular Forces in Materials Science and Drug Design
Intermolecular forces determine the properties of materials, influencing their strength, elasticity, and melting point. By understanding and manipulating these forces, scientists can design materials with specific properties for various applications. In drug design, intermolecular forces affect the solubility, bioavailability, and efficacy of drugs, guiding the development of more effective treatments.
Chemical Kinetics in Industrial Processes and Environmental Monitoring
Chemical kinetics helps optimize industrial processes by controlling reaction rates and yields. It is used in the production of chemicals, pharmaceuticals, and fuels. In environmental monitoring, chemical kinetics is essential for understanding the fate and transport of pollutants, enabling the development of strategies to mitigate their impact.
Acids and Bases in Medicine and Agriculture
Acids and bases play vital roles in medicine and agriculture. In medicine, they are used to regulate pH levels, treat digestive disorders, and fight infections. In agriculture, acids and bases are employed as fertilizers, soil amendments, and pesticides.
Essential FAQs
What is the significance of Fiveable AP Chem Unit 3?
Unit 3 is crucial as it provides a foundational understanding of intermolecular forces, solutions, chemical kinetics, chemical equilibrium, and acids and bases. These concepts are essential for comprehending the behavior of matter and chemical reactions.
How do intermolecular forces influence physical properties?
Intermolecular forces determine the strength of the interactions between molecules, which in turn affects properties such as melting point, boiling point, and solubility. Stronger intermolecular forces lead to higher melting and boiling points, while weaker forces result in lower melting and boiling points.
What are the key factors that affect reaction rates?
Reaction rates are influenced by factors such as temperature, concentration of reactants, surface area of reactants, and the presence of a catalyst. Understanding these factors is essential for predicting and controlling the rate of chemical reactions.
What is the concept of chemical equilibrium?
Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at the same rate, resulting in no net change in the concentrations of the reactants and products. Equilibrium is dynamic, meaning the reactions continue to occur, but the concentrations remain constant.
How are acids and bases defined according to different theories?
The Arrhenius theory defines acids as substances that produce H+ ions in water, while bases produce OH- ions. The Bronsted-Lowry theory defines acids as proton donors and bases as proton acceptors. The Lewis theory defines acids as electron-pair acceptors and bases as electron-pair donors.